GFP Magnetofluorescence
This project is maintained by Maria Ingaramo in the York lab, and was funded by Calico Life Sciences LLC
Research article
Magnetic control of GFP-like fluorescent proteins
*Permanent email: andrew.g.york+magnetofluorescence@gmail.com
†Permanent email: maria.del.mar.ingaramo+magnetofluorescence@gmail.com
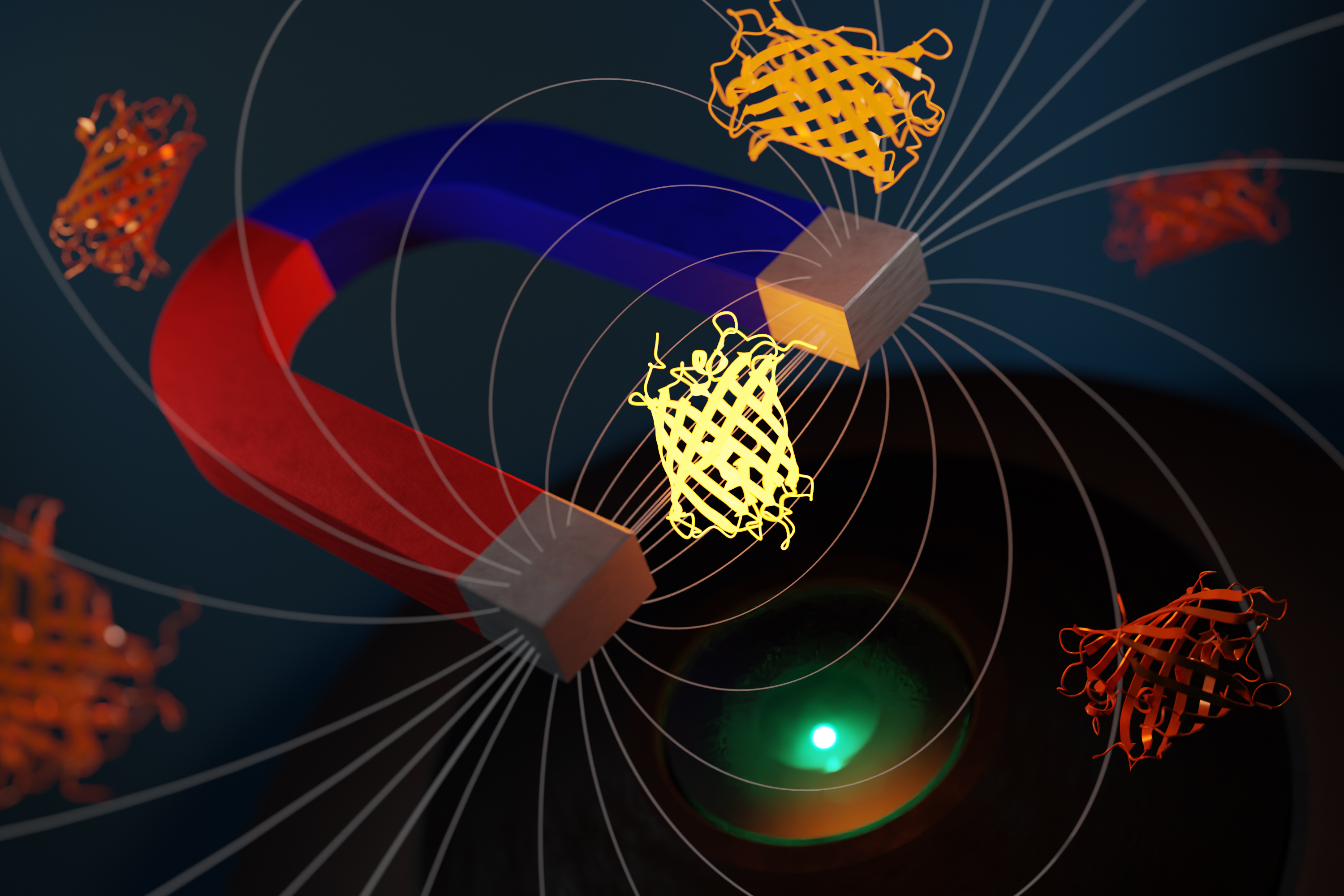
Abstract
We've discovered a simple, nontoxic, biocompatible way to control the brightness of GFP-like fluorescent proteins via modest magnetic fields (~10 mT). Fluorescent proteins which seem magnetically inert (e.g. EGFP, mScarlet) become magnetoresponsive in the presence of an appropriate cofactor (e.g. EGFP-FlavinTag, or an mScarlet/FMN solution). This method works at room-temperature and body-temperature, in vitro, in E. coli and in cultured mammalian cells.
The GFP-family magnetoresponse is weak (\(\Delta F / F \approx 1\%\)), but shows the hallmarks of evolvability. This suggests exciting technological possibilities, both short-term (e.g. lock-in detection, multiplexing) and long-term (e.g. optically-detected MRI, magnetogenetics).
We've also discovered weak magnetoresponse from a member of the LOV-domain family. This suggests the possibility that magnetoresponse is a general feature of fluorescent proteins, and not unique to the cryptochrome/
Peer review status
Pre-print published: July 11, 2023
Aspects of this work have been reproduced independently by the Cohen lab and the Maurer lab; please contact them for details.
Cite as: doi:10.5281/zenodo.8137174
In memory of George H. Patterson, Andrew and Maria's deeply missed friend and advisor. For having known his creative mind and his caring heart, we will always be thankful.
Note that this is a limited PDF or print version; animated and interactive figures are disabled. For the full version of this article, please visit https://andrewgyork.github.io/gfp_magnetofluorescence
Introduction
Proteins that interact strongly with electromagnetic fields give us incredible power to reveal and control biology. This “toolbox” is especially mature at optical frequencies, built from a small set of ancestral proteins that evolved to respond to light (e.g. opsins, the LOV domain, GFP) [Zhang 2011, Chapman 2008, Tsien 1998]. Almost any process (e.g. transcription, oligomerization) or quantity (such as pH or voltage) in a transparent model organism (like yeast, C. elegans, HeLa cells) can be measured or manipulated by a highly-engineered derivative of these optically-active ancestors [Zhao 2022, Schoof 2021, Kennedy 2010, Lazzari-Dean 2022, Berry 2020, Hochbaum 2014].
Unfortunately, mammals are optically opaque, and optogenetic tools only work in our most superficial tissues. We’re exquisitely transparent at DC or RF frequencies, but the “magnetogenetic toolbox” isn’t just immature - it’s nonexistent. Are there ancestral proteins that respond strongly to low-frequency electromagnetic fields? If so, can we engineer these ancestors, and expand the optogenetic revolution beyond yeast, worms and cells, into a magnetogenetic toolbox for flies, mice, dogs - and humans? A candidate ancestor for our toolbox must be robustly electromagnetically responsive in nontoxic body-temperature liquid water, and this response must control downstream (opto)genetic machinery.
We first considered the cryptochrome/
We feared the magnetoresponse of organic molecules might be inherently weak - after all, the decoupling of spin from a molecule's electronic, vibrational, rotational, or conformational dynamics is what makes MRI so powerful. We were therefore shocked to find a paper [Lee 2011] that showed a staggering ~80% change in a simple organic fluorophore’s brightness, in response to a handheld magnet, at room temperature!

This powerful, robust magnetofluorescent response could easily control downstream optogenetic machinery via FRET. Unfortunately, the fluorophore doesn't work in aqueous solvent. Nonetheless, we were inspired - maybe no protein displays this physics, but it’s not insane to hope one could.
Perhaps CRY/PHL's weak, fragile magnetoresponse is unique, and we should begin the daunting process of engineering them into more robust magnetogenetic tools. However, if protein magnetoresponse is common but simply underappreciated, we could save countless years of engineering effort by finding a better starting point. Inspired by Lee et al's demonstration of powerful magnetofluorescent response, we decided to look for magnetoresponse from the family of GFP-like fluorescent proteins. GFP is arguably the single most-studied, most-engineered, and most-used protein known to science. If we can build magnetogenetics from the foundation of GFP, we immediately connect to a vast body of existing knowledge, experience, and innovation.
But does GFP respond in any way to magnetic fields? To our surprise and delight, it does.
Results
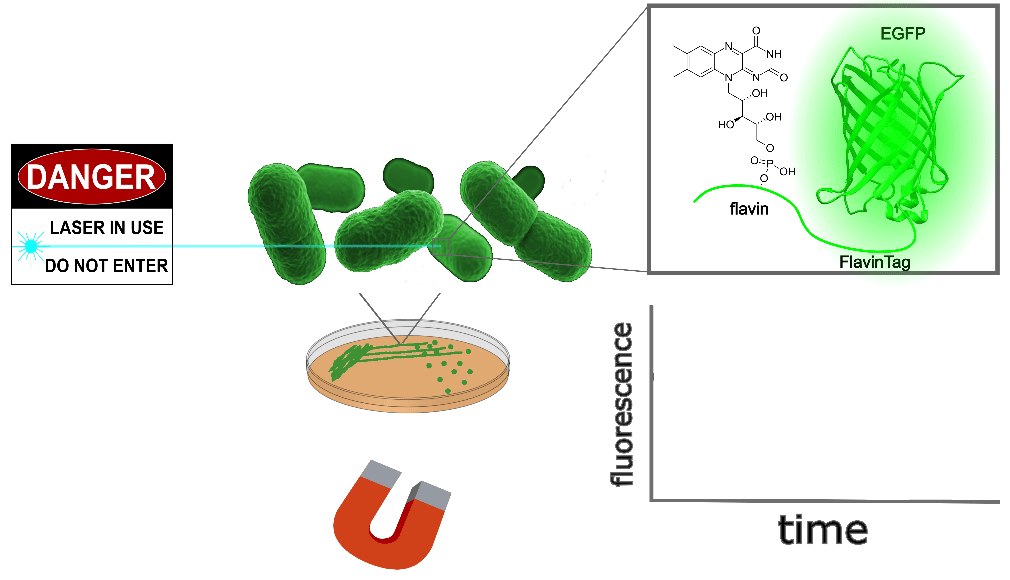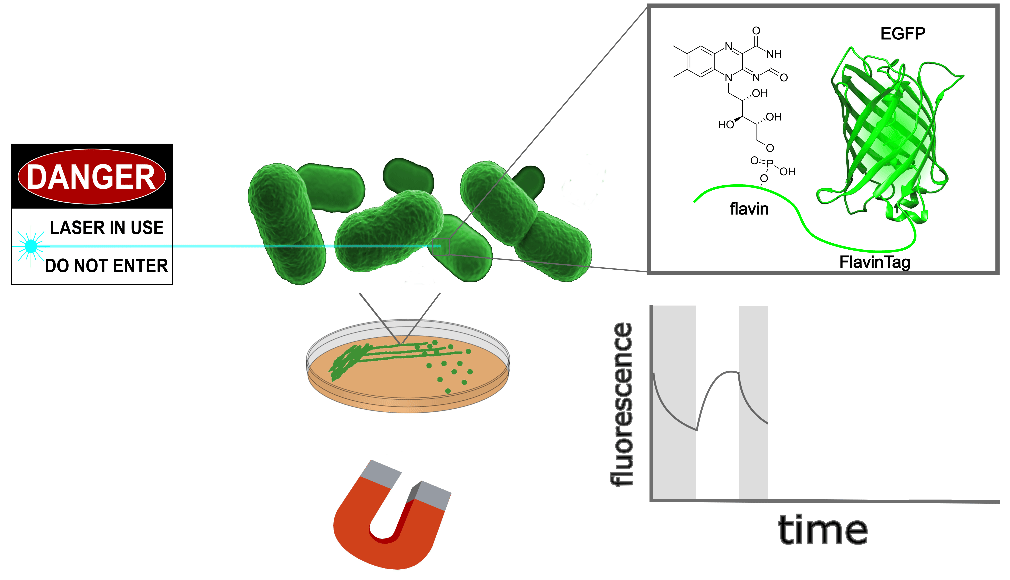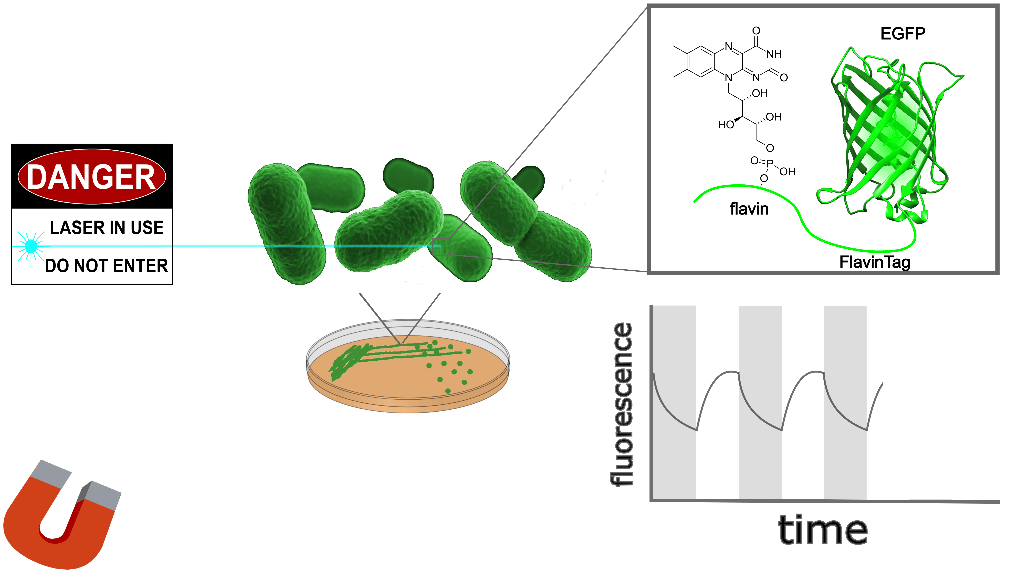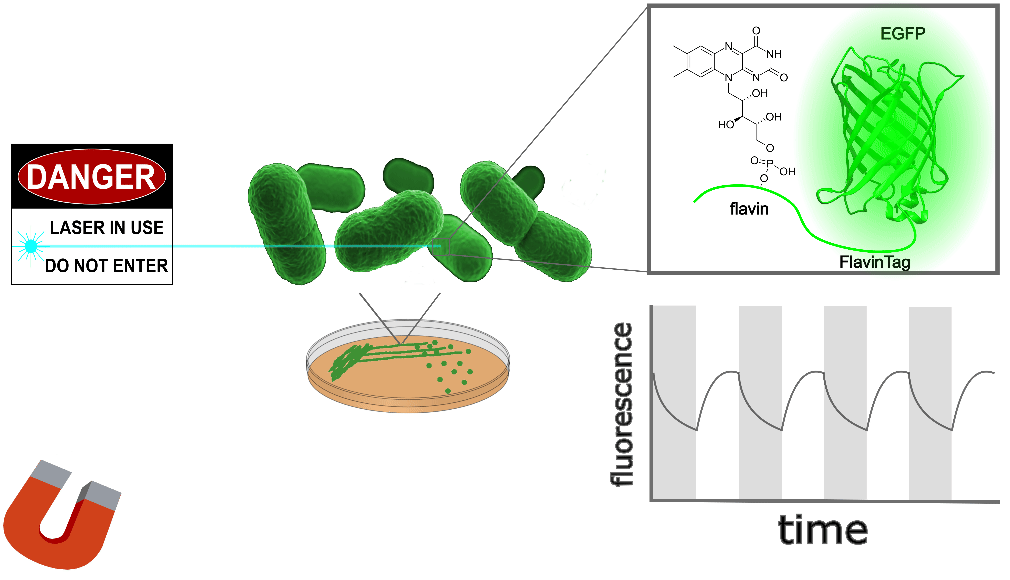
The fluorescence intensity of EGFP responds to magnetic fields in E. coli
We first noticed magnetofluorescent response from E. coli expressing EGFP, using a simple epifluorescence microscope with a servomotor-mounted magnet above the sample. The fluorescent brightness varied slightly but unmistakably as the field alternated from ambient to ~25 mT. Of course, delightful but unexpected results are almost always artifacts, so we began debugging.
Pure proteins are simpler to debug than bacteria, but to our surprise, when we purified the EGFP, the magnetofluorescent effect disappeared (Figure 1 with Sample: EGFP alone selected). Neither purified EGFP nor EGFP-negative bacterial lysate displayed measurable magnetofluorescence, but mixing the two restored the effect. Filtering <7 kDa molecules out of the lysate eliminated magnetoresponse, so we suspected some small molecule(s) in the lysate were acting as cofactors for EGFP magnetofluorescence.
On a hunch, we screened a variety of metals, metabolites, and vitamins as potential cofactors for EGFP magnetoresponse. After a long string of negative results, we finally found a weak hit from an EGFP/riboflavin mixture, which inspired us to try other (more soluble) flavins. A mixture of purified EGFP and flavin adenine dinucleotide (FAD) yielded an exciting result: the EGFP fluorescence intensity varied by ~1% in response to a ~10 mT field, in room-temperature, nontoxic aqueous buffer! (Figure 1 with Sample: EGFP plus FAD photoproduct in vitro selected).
EGFP-FlavinTag: a candidate ancestor for the magnetogenetic toolbox
FAD is an ideal cofactor for our purposes. Flavins aren't just nontoxic - they're ubiquitous, already present in most cells and tissues. Even better, we can use the FlavinTag system
[Tong 2021]
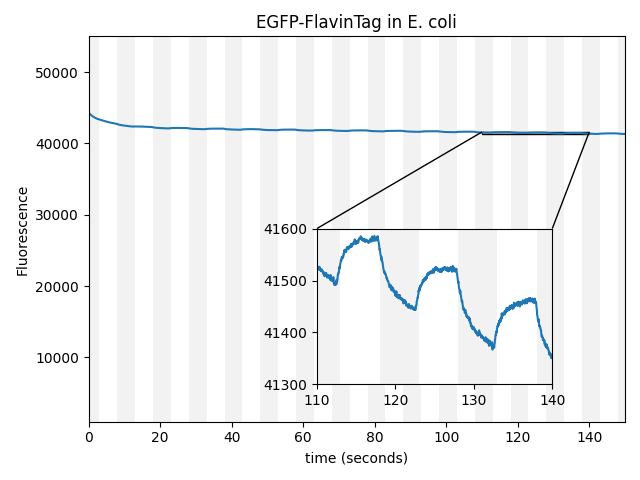
to covalently bind flavin to EGFP, producing a simple genetically-expressed biocompatible magnetically-controlled fluorescent molecule (Figure 1 with EGFP-FlavinTag in E. coli selected). From an engineering perspective, this is an excellent candidate ancestor for magnetogenetic tools: a bright, nontoxic, environmentally robust fluorophore suitable for controlling downstream optogenetic machinery via FRET, building on the vast and mature body of GFP-related technology. The intensity modulation (~0.25% at ~25 mT) is much weaker than we'd prefer, but our construct is completely unoptimized for magnetic response, and
[Lee 2011]
gives us hope that this could improve substantially with further engineering.
| Sample: |
EGFP-FlavinTag is magnetoresponsive in vitro, and enzymatic removal of the FlavinTag eliminates the magnetoresponse as expected. As shown in Figure 1, EGFP-FlavinTag fluorescence is also magnetoresponsive in living bacteria. Note that while E. coli lysate inspired our discovery of EGFP/flavin magnetofluorescence, this does not mean that flavin was the (only) cofactor in the lysate; we suspect there are additional cofactors in E. coli that we have yet to discover.
Flavins are also the cryptochrome (magneto)fluorescent cofactor. This is highly suggestive, but we don't need flavin; we found several other small molecules which yield magnetofluorescent response when mixed with EGFP. As shown in Figure 1 for various choices of Sample:, hydroxykynurenine, 3-hydroxyanthranilic acid, cinnavalininate, and the tetrazoleum viability stain WST-8 all function as EGFP magnetofluorescence cofactors in vitro, although we find them less convenient than flavin. None of these cofactors yield measurable magnetoresponse in the absence of EGFP (e.g. Figure 1 with Sample: FAD alone), nor does FAD mixed with a non-fluorescent EGFP ∆Y66 mutant. We don't know how to predict which molecules will work as magnetofluorescence cofactors, but even our limited observations share features: small molecules with one or more aromatic rings that participate in electron transfer and/or tryptophan metabolism
[Ishiyama 1997,
Farmer 2017,
Nakazawa 2009].
The fluorescence intensity of EGFP-FlavinTag responds to magnetic fields in vivo
Our ultimate goal is to enable magnetogenetics in mammals, so we tested EGFP-FlavinTag in cultured mammalian cells. As illustrated conceptually in Figure 2, the setup is similar to our E. coli work, except now the EGFP-FlavinTag is expressed in COS7 cells and targeted to the interior of the mitochondria via a COX8A localization sequence.
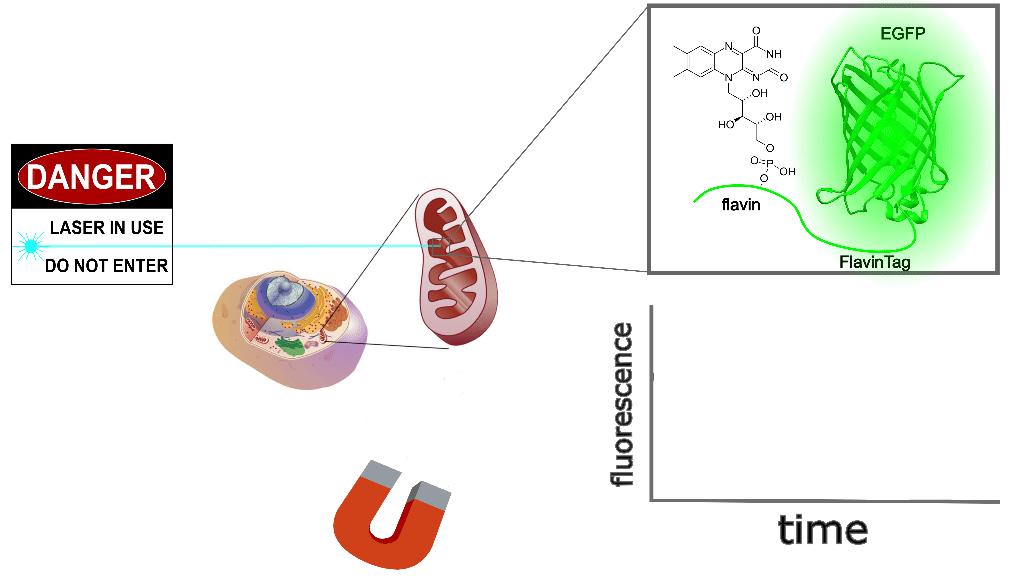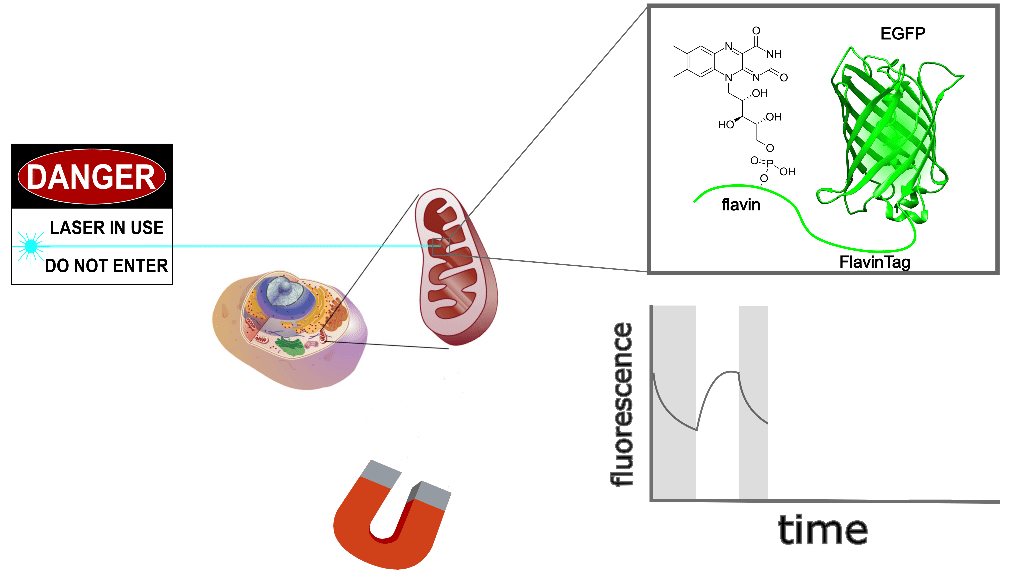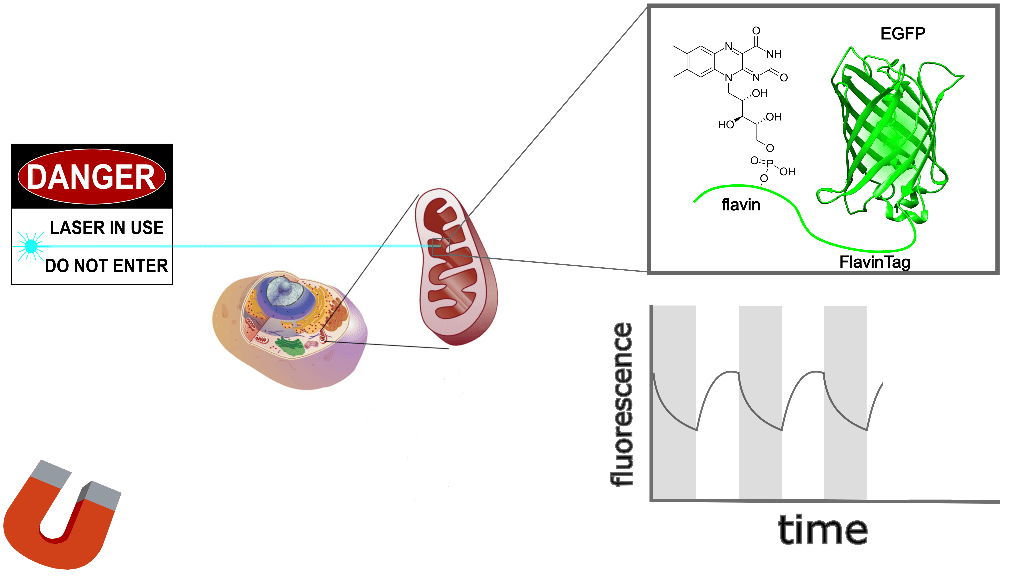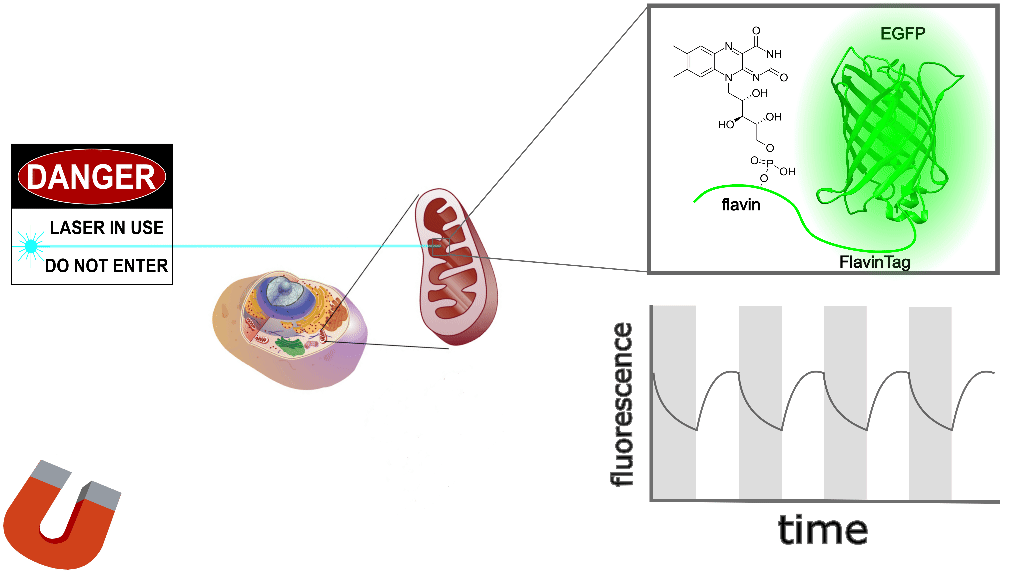
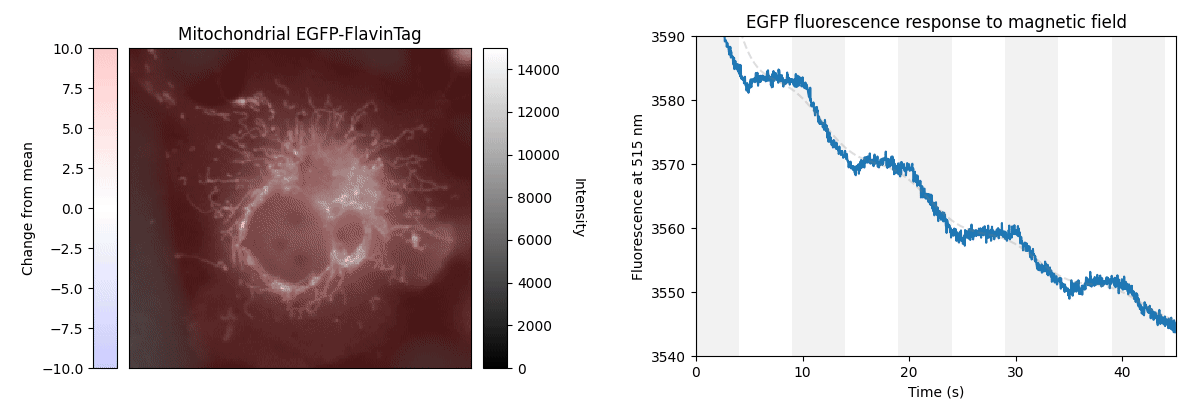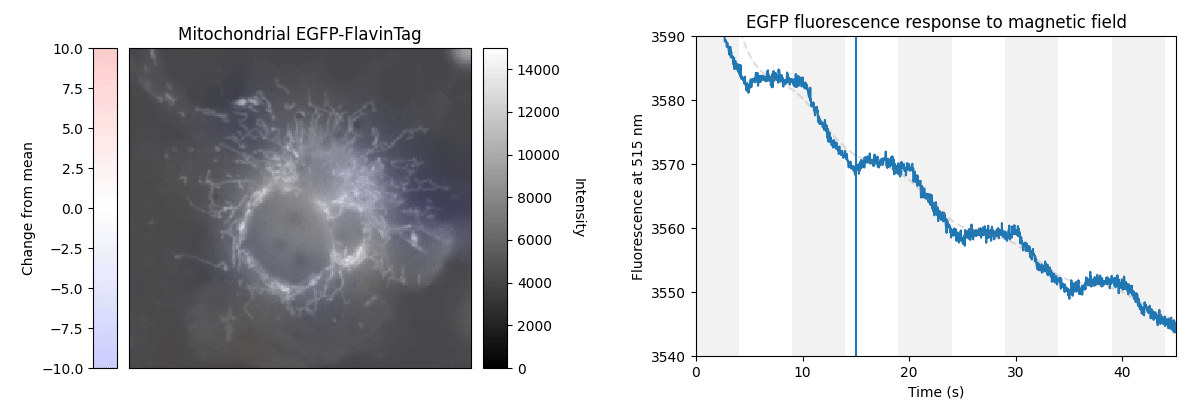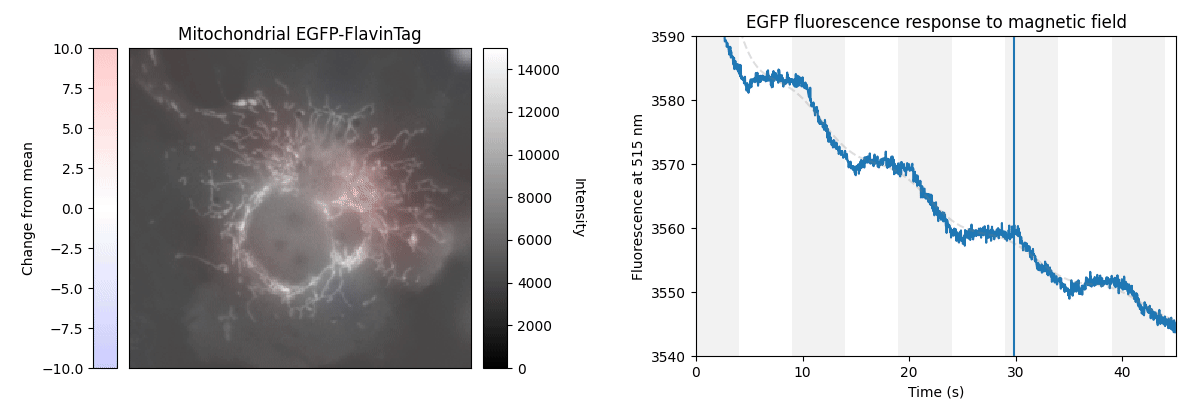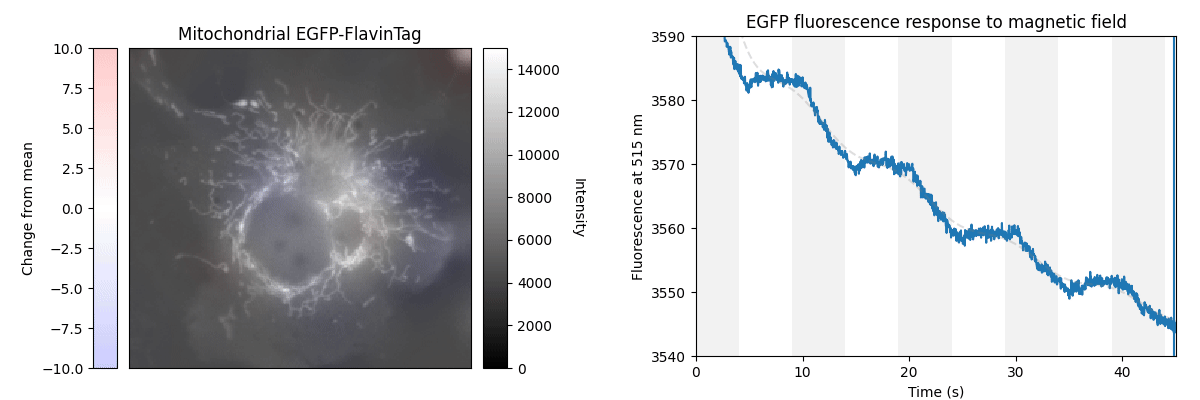
The GFP family is fluorescent in a remarkably wide range of conditions, which is crucial for their utility. Nonetheless, the detailed fluorescent properties of the GFP family (e.g. brightness, lifetime, bleaching/switching rates) depend on their environment (e.g. pH, oxygen concentration, temperature) in a rich, complex way. We assume the magnetoresponse of EGFP-FlavinTag also depends on its environment in a rich and complex way, but it must retain at least some magnetoresponse in mammalian cells to be useful for our purposes.
The magnetoresponse in COS7 cells (Figure 2) is different from E. coli (Figure 1), but magnetoresponse is clearly present. We suspect the magnitude of the magnetoresponse could be enhanced by extra flavin, perhaps via coexpression of FAD synthase [Tong 2022]. Traditionally, GFP's environmental dependence is either minimized via engineering to yield tags, or accentuated and/or characterized to yield biosensors. We expect both approaches will be similarly fruitful for magnetoresponsive proteins like EGFP-FlavinTag. For now, though, we're simply happy that it seems to work at all in mammalian cells.
Magnetoresponse may be a generic feature of GFP-like fluorescent proteins
We observed at least some magnetoresponse in E. coli from several other GFP-like fluorescent proteins, including mVenus, tdVenus, dsRed, and mScarlet. From a scientific perspective, magnetoresponse seems far more common than we expected, which raises countless questions beyond the scope of this manuscript. What is the mechanism of magnetoresponse? What aspects of each fluorophore determine its magnetoresponsive behavior? What are the physical limits on this response? From an engineering perspective, our diverse starting points show the hallmark of evolvability: magnetoresponse is neither insensitive to, nor intolerant of minor modifications (GFP→mVenus) and major modifications (GFP→mScarlet) to the protein sequence. We also hope that a diverse palette of absorption/emission colors will allow us to (eventually) connect a wide range of chemiluminescent donors to a wide range of optogenetic acceptors via a magnetoresponsive BRET-acceptor/FRET-donor intermediate [Carriba 2008, Takai 2015, Lee 2023].
Purified mScarlet yields the largest protein magnetoresponse we've observed, almost 3% when mixed with a flavin mononucleotide (FMN) photoproduct in vitro, as shown conceptually (Figure 3) and quantitatively (Figure 4) below. mScarlet is arguably the best red fluorescent protein ever engineered, so we're especially excited that such a promising foundation yields such a large magnetoresponse [Bindels 2017]. We also expect red fluorophores like mScarlet will be especially important for magnetogenetics with external illumination or emission, because longer-wavelength light typically penetrates better in translucent tissues.
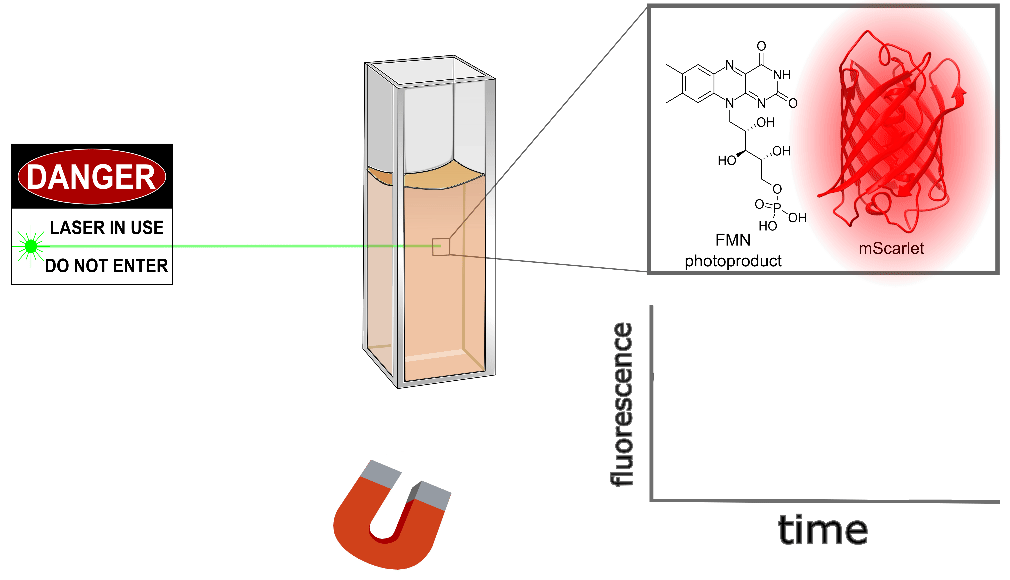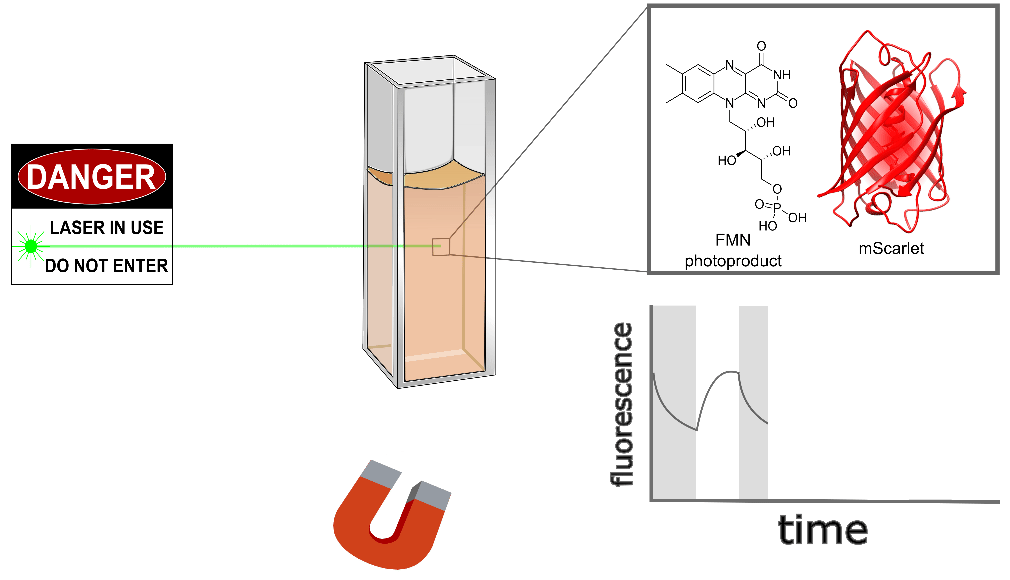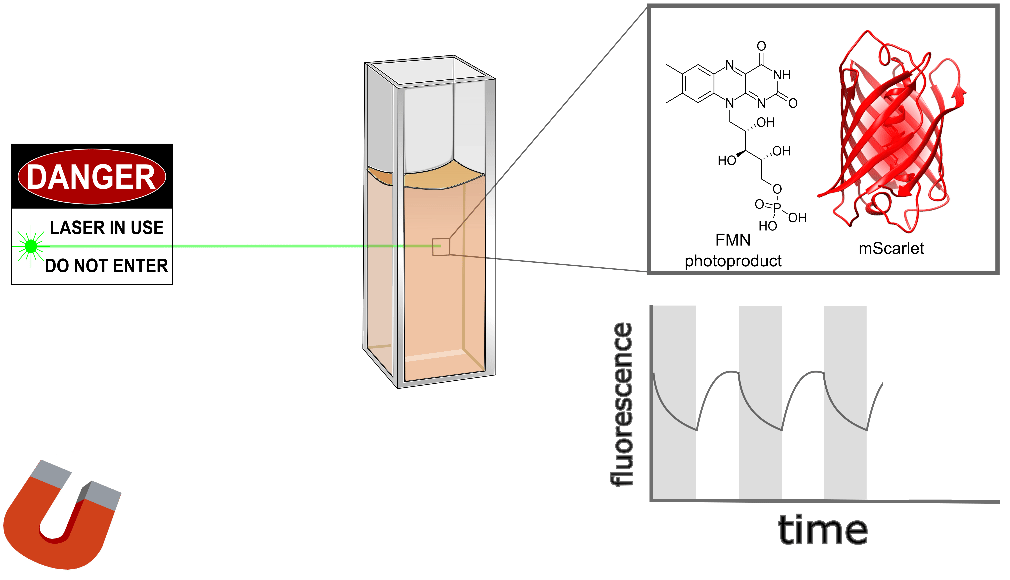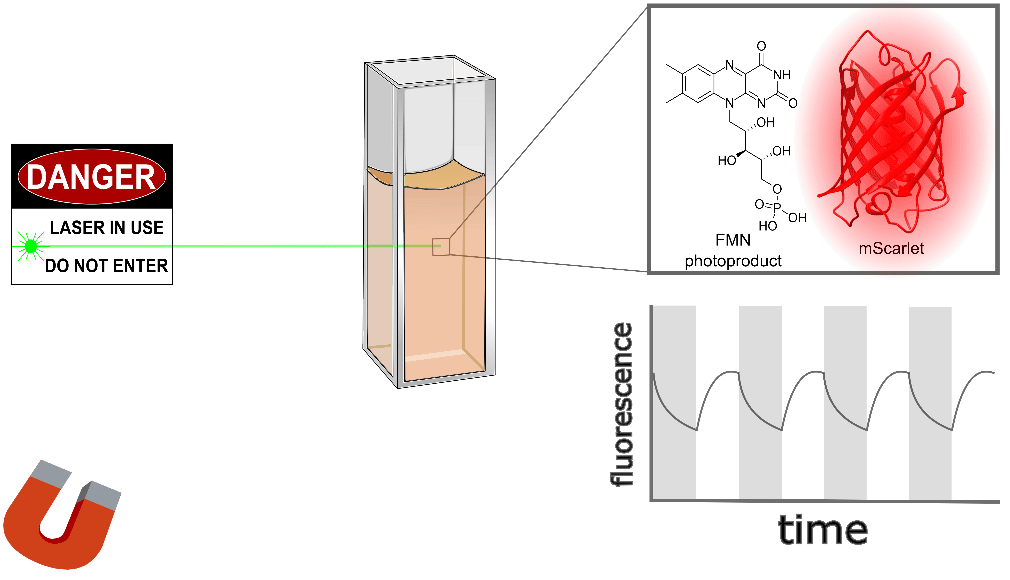
| Sample: |
Like EGFP, we also found non-flavin small molecules which yield magnetofluorescent response when mixed with mScarlet. As illustrated in Figure 3 and shown in Figure 4 for various choices of Sample:, cinnavalininate and WST-8 function as mScarlet magnetofluorescence cofactors in vitro. None of its cofactors yield measurable magnetoresponse in the absence of mScarlet, nor does purified mScarlet in the absence of a cofactor (e.g. Figure 4 with Sample: FMN alone... and Sample: mScarlet alone...). Interestingly, several cofactors that yield EGFP magnetoresponse don't seem to work with mScarlet (FAD, hydroxykynurenine, 3-hydroxyanthranilic acid), nor does FMN yield measurable magnetoresponse when mixed with EGFP. Neither mScarlet-FlavinTag nor FlavinTag-mScarlet fusions showed measurable magnetoresponse, but we intend to keep trying (e.g. by varying the FlavinTag linker). In contrast, EGFP-FlavinTag worked on our first try, but we suspect we just got lucky. We have no model to explain any of these observations, but we hope some of our readers are qualified and inspired to tackle this question (and the many other questions raised here).
Unlike EGFP, the excitation/emission spectrum of mScarlet is substantially different from flavin, which gives us a convenient way to dissect the photophysics of flavin cofactors a bit more. Cyan illumination (~470 ± 12 nm) preferentially excites flavin, while green illumination (~550 ± 7.5 nm) preferentially excites mScarlet, and our emission filter (705 ± 36 nm) preferentially transmits mScarlet's emission. As shown below, this suggests that a long-lived photoproduct of cyan-illuminated FMN is the magnetofluorescent cofactor for mScarlet, rather than FMN itself.
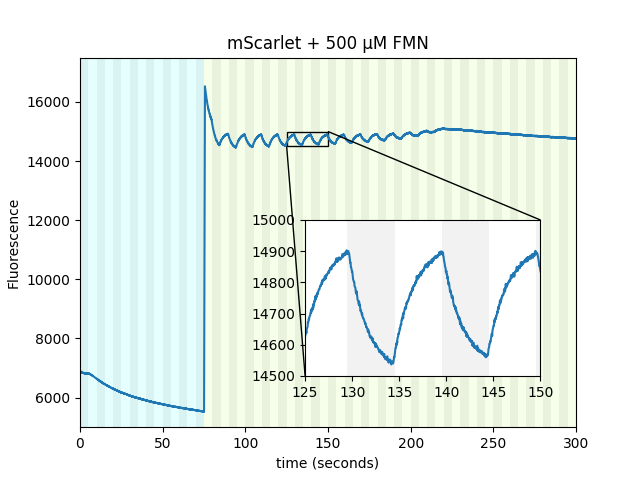
| Sample: |
Our non-flavin cofactors don't require cyan illumination to yield mScarlet magnetoresponse, nor does mScarlet in E. coli. However, purified mScarlet mixed with FMN yields almost ~3% magnetofluorescent response in the green/red channel (550 nm excitation, 705 nm emission), if and only if it's pre-illuminated with cyan light (Figure 4 with Sample: mScarlet + FMN with/without 470 nm pre-excitation). There is measurable fluorescence but no apparent magnetoresponse in the cyan/red channel during pre-illumination (470 nm excitation, 705 nm emission). Green/red-channel magnetoresponse still occurs even if several minutes elapse between cyan illumination and green/red-channel interrogation, suggesting a long-lived cyan-generated photoproduct. We suspect the cyan-generated photoproduct is from FMN rather than mScarlet, based on their absorption spectra, and we tentatively speculate that the long-lived state is an FMN radical. We suspect that similar photophysics occurs for EGFP-FlavinTag and EGFP/FAD mixtures, but the overlapping EGFP/flavin absorption/emission spectra obscure this dynamic.
Magnetoresponse may be a generic feature of fluorescent proteins
We observed small but unmistakable magnetoresponse in E. coli from the LOV-family protein AsLOV C450A V416T [Lungu 2012]. This was the first (and only) LOV-family protein we checked, so we were quite surprised to get such an easy "hit" from essentially a shot in the dark.
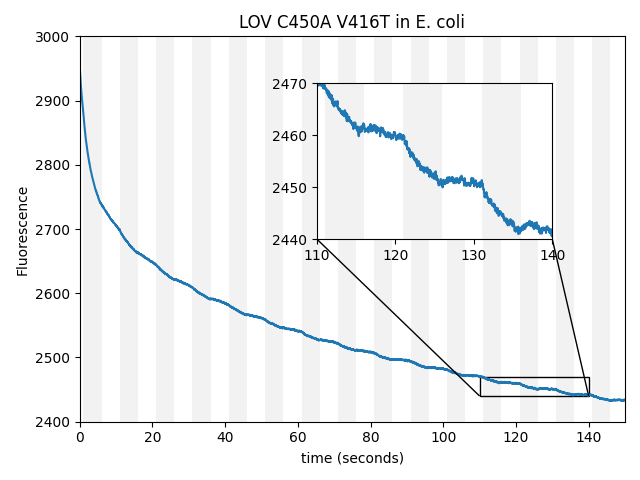
Remarkably, we've found magnetoresponse in every family of fluorescent proteins we've checked so far (CRY, GFP, and LOV). The CRY/PHL family is arguably the most well-known and well-studied magnetoresponsive fluorophore, but in our hands, also the weakest. From a scientific perspective, this raises fascinating questions. Are most (all?) fluorescent proteins magnetoresponsive, given the right cofactor(s)? If so, why has the field focused almost exclusively on CRY as the putative origin of animal magnetosensation? Are proteins generally magnetoresponsive, but we only notice the fluorescent ones because magnetofluorescence is easy to measure? From an engineering perspective, we're excited by the possibility that LOV-family proteins can be magnetically modulated, since they already form the foundation of so many successful optogenetic tools, and have already been "powered" via BRET [Lee 2023]. We only checked for magnetofluorescent response, but it would be extremely useful if LOV-family magnetoresponse also directly affected conformation or dimerization. Depending on the strength, sturdiness, and evolvability of GFP-family vs. LOV-family magnetoresponse, the LOV family may prove to be a compelling candidate ancestor for magnetogenetics.
Discussion
The central scientific question raised by our observations is obviously: How and why do fluorescent proteins respond to magnetic fields? Every paper we've read that discusses magnetoresponsive proteins and/or fluorophores explains the phenomenon in terms of the "radical pair" mechanism [Schulten 1976]. Like all of these papers, our simple observations here neither prove nor disprove the relevance of this model. We're certainly eager to understand the mechanism behind our observations, and we're happy to have a candidate mechanism, but for now we prefer ignorance over misplaced confidence.
The central technological question raised by our observations is obviously: What are magnetoresponsive fluorescent proteins good for? In the short-term, a magnetically-switchable fluorophore enables "lock-in detection", meaning a signal that blinks over a background that doesn't, yielding accurate background subtraction. Our magnetoresponse also has a finite equilibration rate, which varies depending on the protein sequence; if this rate is sufficiently independent of the environment, it allows multiplexing, and if not, it allows biosensing [Peksağlam Seidel 2021]. Longer term, if magnetically sensitive fluorescent proteins display magnetic resonance [Carbonera 2009], perhaps gradient fields and tuned microwave/RF pulses would allow MRI-like imaging with optical readout in translucent samples or spatial confinement of magnetogenetic control.
All of these questions require deep expertise and years of hard work. The questions raised here fall at the intersection of quantum mechanics, biochemistry, invention, and engineering, and will need input from almost every scientific discipline. Revealing and understanding the GFP magnetofluorescent mechanism needs quantum chemists, transient absorption spectroscopists, ESR and NMR experts. Enhancing the magnitude and robustness of GFP magnetoresponse needs protein engineers, structural biologists, biochemists, and biophysicists. Integrating magnetoresponsive proteins into useful magnetogenetic circuits needs inventors, bioengineers, and biologists. We hope we've inspired you to consider taking up part of this work; we certainly can't do it alone.
Author Contributions
R.F.H. and A.R. performed experiments. J.R.L.D. contributed to data interpretation and experimental design. A.G.Y. wrote the manuscript, interpreted data, and contributed to conceptualization of the project. M.I. conceptualized and designed the project, performed experiments, interpreted data and drafted the manuscript.Acknowledgements
We thank Alfred Millet-Sikking for help constructing the magnetic field control set up, Ksenia Bravaya for directing us to emap, and Shaun Burd for help with controlling electromagnets. We thank Justin Caram, Yongjia He, Clarice Aiello, Adam Cohen, Hohjai Lee, Dongcheol Park, Daniel Kattnig, Ashok Ajoy, Zachary Jones, the Kasevich lab, Steven Vogel, Steven Boxer, Srijit Mukherjee, Alessandro Agostini, Andrea Volpato, David Garrett, Peter Maurer, Uri Zvi, Jacob Feder, Ben Soloway, Tim Craggs, Namrata Anand, and Jenny Clark for thoughtful discussions and advice.
Open-source software
As always, our work here depends critically on the open-source software community. Among our crucial dependencies:
- Python
- Numpy [Harris 2020]
- Scipy [Virtanen 2020]
- Matplotlib [Hunter 2007]
- pySerial
- tifffile
- ImageJ [Schneider 2012]
- Micro-Manager [Edelstein 2010]
- FFmpeg
We sincerely thank the authors of these projects for enabling our work.