Protein Magnetofluorescence
This project is maintained by Maria Ingaramo in the York lab, and was funded by Calico Life Sciences LLC
Appendix
Note that this is a limited PDF or print version; animated and interactive figures are disabled. For the full version of this article, please visit https://andrewgyork.github.io/gfp_magnetofluorescence
Magnetic control of the brightness of fluorescent proteins
Raw data
The raw data used to generate the main text figures is available at doi.org/10.5281/zenodo.8136926 and doi.org/10.5281/zenodo.8137092
Instrumentation
We used a Leica DMI8 microscope with a 10x objective and a SpectraX light source to deliver 1.7 ms of 1.1 W/cm2 power per camera exposure through a 470/24 nm excitation filter, or 1.4 W/cm2 per camera exposure through a 550/15 nm excitation filter every 30 ms. We used the same quad dichroic (Leica) for all experiments and either a 540/21 nm emission filter to image green proteins (like EGFP) or a 705/72 nm filter to image red proteins (like mScarlet). In a typical experiment, we move a 1 cm3 N42 neodymium magnet adjacent to/distant from the sample every 5 seconds using a servo motor (HS-422) while acquiring 1.7 ms camera exposures every 30 ms, continuously on a PCO edge 4.2 camera with 4x4 pixel binning. The magnet moved relatively quickly (<<1 s) controlled by an Arduino UNO, and modulated the magnetic field at the sample between ~0 to ~10 mT (or ~25 mT for E. coli samples). The magnet movement and the acquisition were not synchronized, but we started the traces when the magnet was close to the sample.
Sample preparation
Fluorescent protein coated beads: To attach fluorescent proteins to Ni-NTA-coated beads, we first transformed T7 Express E. coli (NEB) with pRSET plasmids that encoded fluorescent proteins with an N-terminal 6xHIS tag and selected on LB/Carbenicillin plates. Because we frequently encounter problems with plasmid loss, we inoculate 1 L of LB/Carbenicillin with all of the transformants and grow the liquid culture for ~3 hours at 37°C. After overnight induction with 200 μM IPTG, samples were pelleted, resuspended, and lysed in 50 mM phosphate buffer pH 8 + 500 mM NaCl with BugBuster. After sample centrifugation, the lysate was incubated with 1 mL of cOmplete Ni-NTA agarose beads (Roche) for 1 hour. The beads were washed 3 times, and stored in 1 mL of 50 mM phosphate buffer pH 8 + 500 mM NaCl. For imaging, we diluted 0.5 μL of this sample into 100 μL PBS (Corning 21-040-cv) in a 384-well glass bottom plate (Cellvis). Flavinated-EGFP beads were made similarly, except that we co-transformed E. coli with pRSET-FP-FlavinTag and a pD881-MR vector (ATUM) encoding the flavin transferase ApbE (pRham-StrepTag-ApbE). Growth was carried out with the addition of Carbenicillin/Kanamycin and 50 μM riboflavin, and we induced first with 0.2% rhamnose overnight and then with 200 μM IPTG for 6 hours the next day. To purify mScarlet with a Streptag instead of a 6xHIS, we used streptactin superflow plus beads (Qiagen) instead of Ni-NTA beads. For imaging beads, we used an additional 1.6x magnification lens on the emission path.
Metabolite screen: We screened a library of 730 metabolites [Hicks 2023] on our microscope, using protein-coated Ni-NTA beads and a 1x mag changer, a quad dichroic, a 470/24 nm excitation filter, and a 540/21 nm emission filter for GFP, and 550/15 nm and 705/72 nm for mScarlet. We also screened both proteins using the corresponding emission filters, with 405, 470, 560 and 640 nm concomitant illumination, but did not get any additional magnetoresponsive hits. The metabolites' final concentration was 700 μM in PBS.
Pure fluorescent protein in solution samples: Purified protein was provided by Wuxi Biortus. 4 μL samples (with final concentrations of 5.1 μM mScarlet or 19 μM EGFP in PBS) were covered with 3 μL mineral oil and imaged in 1536-well glass bottom plates. We used 1536-well glass bottom plates because the field-of-view (FOV) covers the whole well, so diffusion at the FOV-edges is less of a concern. Concentrations were determined using extinction coefficients of 55900 M-1cm-1 at 488 nm for EGFP, 100000 M-1cm-1 at 569 nm for mScarlet, and 11300 M-1cm-1 at 450 nm for FAD and FMN. For the other metabolites, we dissolved the powder in either water or DMSO to make 50 mM stocks, depending on their solubility.
Mammalian cell experiments: We transfected Cos7 cells with pCDH plasmids expressing mito-EGFP-FlavinTag and a flavin transferase separated by a T2A sequence in a glass bottom 8-well chamber (Cellvis). Samples were imaged ~48 hours after transfection, using a 40x objective, a 470/24 nm excitation filter, and a 515/30 nm emission filter. The DMEM + 10% FBS (with added glutamax and antibiotics) was replaced with warm imaging solution (Invitrogen A14291DJ) without any additives right before acquisition. The whole microscope was warmed up to 37°C before the start of the experiment, but the incubator turned off while imaging to avoid vibrations. Samples were imaged without supplying CO2 within an hour of media exchange.
We also transfected EGFP-FlavinTag without a flavin transferase, as well as EGFP without a FlavinTag, both in the cytosol and mitochondria. The magnetic field effect on the fluorescence was ambiguous in these samples. We did not perform any optimizations like adding exogenous flavin to the media, establishing the localization of the ApbE enzyme, or adding a gene for overexpression of a flavin synthase [Tong 2022]. We suspect that optimization of these parameters is quite important; just because we didn't see magnetoresponse in these samples doesn't mean it wasn't there.
Experimental design
All the experimental results presented in the main text, unless stated, are performed with protein immobilized on Ni-NTA beads. These experiments are performed in 100 μL of the relevant cofactor/PBS solution in a 384-well plate (Cellvis), and the FOV is a small fraction of the whole volume. Experiments with purified protein in solution are performed in 1536-well plates, which have wells that fit entirely within the FOV. All in vitro experiments use ~0 to ~10 mT magnetic field changes. However, E. coli samples allowed us to get the magnet closer, so the magnetic field changes between ~0 and ~25 mT. If no concentration is specified, it means that the experiments contain 5.1 μM mScarlet, 19.1 μM EGFP, 1000 μM FAD or 500 μM FMN in PBS. We carried out experiments at room temperature, except those using mammalian cells, which were at 37°C.
We would like to address some of the experimental pitfalls that we encountered while looking for magnetic field effects on fluorescence. First, reflection from the magnet produced a very fast and large change in signal that can be mistaken for a magnetic field effect, but this can be avoided by covering the sample with black matte paper. Second, we used an electromagnet to corroborate that we can qualitatively obtain similar traces. This indicates that vibrations from the servo movement or heating from the electromagnet are not likely explanations for the observed behavior. Third, we used a long working distance (~1.1 cm) air objective to avoid contact with the sample, and reduce the magnet's ability to pull on the objective. The traces are similar whether we use hardware autofocus or not.
We'd also like to highlight a few observations/tips for those interested in repeating our results. Start with a 3 to 5 minute timelapse at the maximum camera framerate and high power, while changing the magnetic field at 5 second intervals. The effects are small and are easy to miss without a repeating pattern to guide the eye. More cofactor is not necessarily better, so testing a few concentrations might be useful. I'd suggest starting with WST-8 (MedChemExpress, HY-D0831), which we dissolved in water to make a 50 mM stock that can be stored frozen at -80°C for at least 6 months. WST-8 works with both EGFP and mScarlet, and unlike flavins, it does not require pre-illumination. To look at mScarlet with flavins, it might be easier to start with 470/560 nm concomitant excitation. It took us a bit of time to get good at observing the modulation, and you might not succeed on the first try. If so, please reach out.
Magnetic field response of fluorescent proteins in E. coli
We screened by looking at T7 Express (NEB) cells expressing fluorescent proteins from a pRSET plasmid. A colony was scraped off the LB/carbenicillin plate two days after transformation and sandwiched between a coverslip and a multiwell plate optical lid (Cellvis) turned upside-down. We applied different amounts of pressure to each sample in order to maintain maximum power while avoiding saturating the camera. We believe that high excitation power is important, because some cofactors, like FMN, seem to require production of a photo-induced intermediate. Since these samples are very flat, we could get the magnet closer to the FOV, which produced a magnetic field of ~25 mT. For the most part, we looked at proteins that we already had on hand, except for dmCry W394F [Zollitsch 2018], an FAD binding protein with a mutation that reportedly increased transient absorption spectra magnetoresponse. Among the proteins that we looked at were mCardinal, mCherry, KillerRed, dsRED, mScarlet, pHuji, mVenus, tdVenus, EGFP, StayGold, mCerulean, CyOFP, LSSmOrange2, mMaple, AsLOV2 V416T C450A, dmCry W394F. AsLOV2 V416T (residues 404-546) [Kawano 2013] is a mutant that we already had, to which we (on a hunch) added the C450A mutation [Kay 2003], which (luckily!) seemed to increase the magnetoresponse.
In the green/yellow family, we noticed magnetoresponse from mVenus, tdVenus, EGFP, AsLOV2 V416T C450A, and dmCry W394F. From the red protein family, we noticed magnetoresponse from mScarlet and dsRed. Interestingly, mScarlet did not require 470 nm excitation which suggests that the endogenous cofactor(s) in E. coli aren't flavins. Other proteins did not show a convincing response, but we do not rule out that a modulation might be observed under more favorable conditions (like lower background, higher endogenous cofactor levels, better matched imaging wavelengths, etc).
We tested tdVenus-coated beads in PBS, which were not magnetoresponsive, but adding FAD restored weak magnetoresponse. However, FAD and ascorbic acid gave a much stronger magnetoresponse [Kattnig 2016]. Because the reaction was very sensitive to relative concentrations of the cofactors, we decided to pursue characterization of EGFP instead, which displayed a strong magnetoresponse without needing ascorbic acid.
We would also like to note that co-expressing a flavin transferase enzyme and adding 50 μM riboflavin dramatically improves the magnetoresponse of EGFP-FlavinTag in E. coli (Figure 1, EGFP-FlavinTag selected).
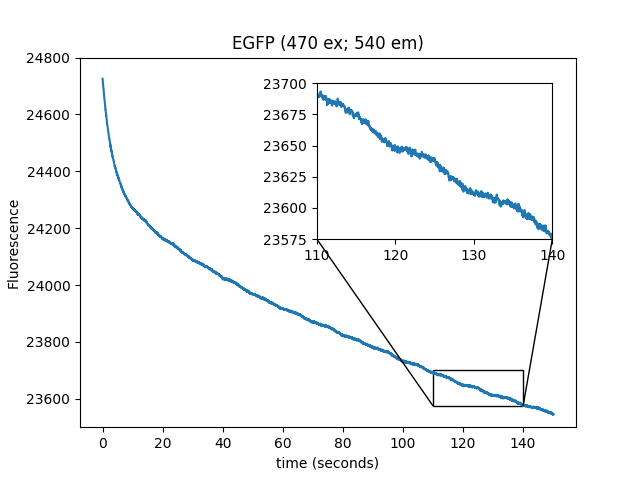
| Fluorescent protein: |
Additional controls using mScarlet immobilized on beads
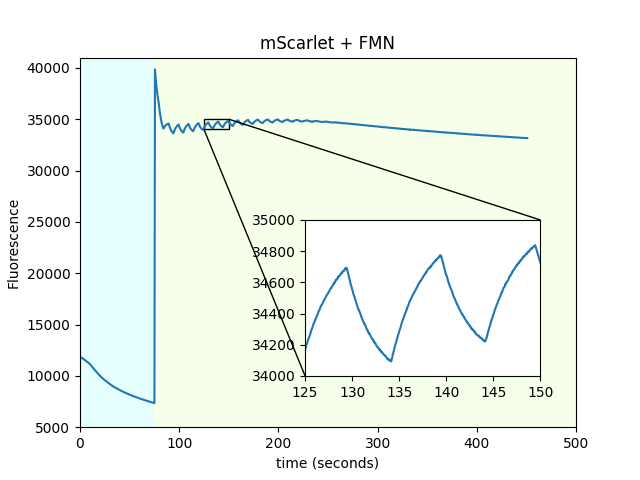
We performed several controls to try to establish that FMN and mScarlet are the source of the magnetic field change in fluorescence. First, to rule out that the nickel metal from the Ni-NTA beads is somehow involved in the magnetic field response, we switched the 6xHIS tag for a StrepTag and the Ni-NTA beads for streptactin-coated beads, and observed similar results. Second, in order to establish that mScarlet is indeed the source of the fluorescent magnetoresponse and it is not merely the flavin accumulating on the bead surface, we deleted tyrosine 68 from mScarlet; as shown below, this eliminates magnetoresponse in the red channel. Deletion of the chromophore tyrosine extinguishes the fluorescence while maintaining the overall protein fold, (at least with mVenus, Steven Vogel, personal communication), and we believe it has the same effect on mScarlet. This experiment also suggests that it is unlikely that we are just observing changes in fluorescence originating only from the cofactor abstracting electrons from the fluorescent protein surface without involvement of the fluorescent protein's chromophore. Also in support of the previous point, we used the FlavinTag to covalently attach a flavin to the C-terminus of mScarlet and also mScarlet ∆Y68 and observed no magnetoresponse. Even though we did not quantify the percent flavination, these beads are visibly yellow, indicating that a substantial fraction of the protein has a flavin attached. Finally, and quite interestingly, mScarlet/FAD mixtures do not exhibit magnetoresponse. A possible explanation is that mScarlet binds FMN, but not FAD.
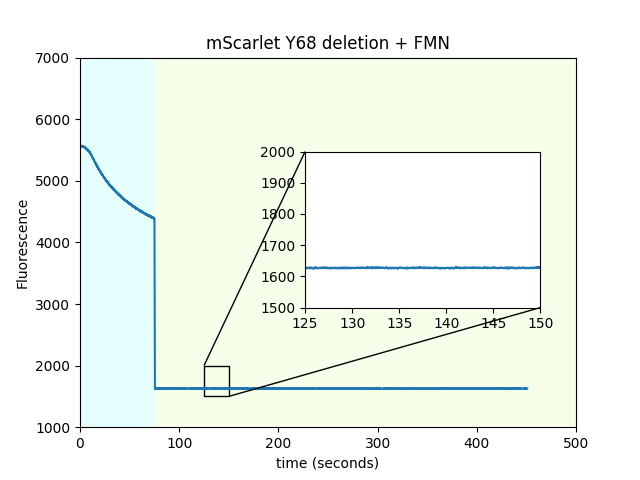
| Sample: |
Additional controls using purified mScarlet in solution
We repeated the experiments from Figure 4 of the main text (which immobilized mScarlet on beads) using pure protein in solution (PBS), in wells small enough to fit in the FOV. This prevents diffusion artifacts from complicating the interpretation of the traces. We observed similar results qualitatively, indicating that the beads are irrelevant to the modulation of fluorescence by the magnetic field, although quantitatively, the amplitude of the response is lower.
| Sample: |
mScarlet tryptophan mutants on beads
We mutated each of the three tryptophans present in mScarlet to probe the hypothesis that one of them would provide a path for electron transfer from the chromophore to the surface of the protein. We found that W144 was the only one that changed the magnetoresponse. However, this mutant produces significantly dimmer beads. We're hesitant to overinterpret this result, but since we have the data, we might as well share it.
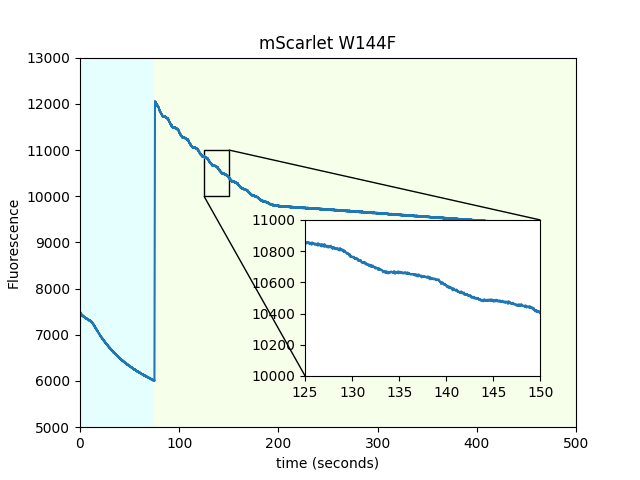
| mScarlet tryptophan mutant: |
Dependence of mScarlet magnetofluorescence on FMN concentration
We used purified mScarlet in solution to determine the fluorescent magnetoresponse as a function of FMN concentration.
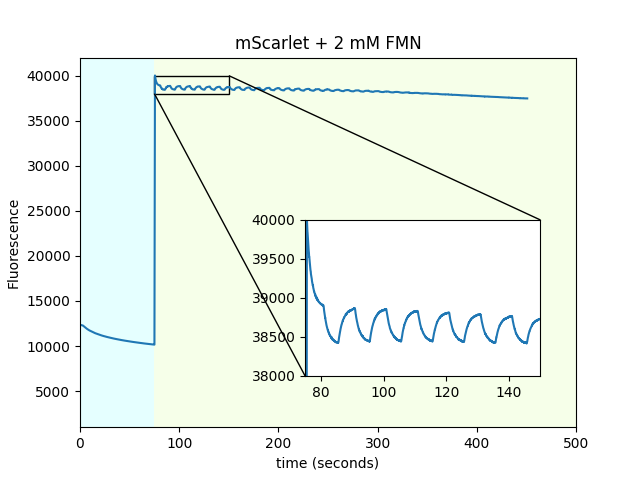
| FMN concentration: |
Dependence of mScarlet magnetofluorescence on 470 nm power
We used purified mScarlet in solution to determine the fluorescent magnetoresponse as a function of 470 nm pre-irradiation power.

| 470 nm pre-irradiation power: |
Dependence of mScarlet magnetofluorescence on 550 nm power
We used purified mScarlet in solution to determine the fluorescent magnetoresponse as a function of 550 nm imaging power. We observed little dependence, other than absolute intensity, and photobleaching rates.
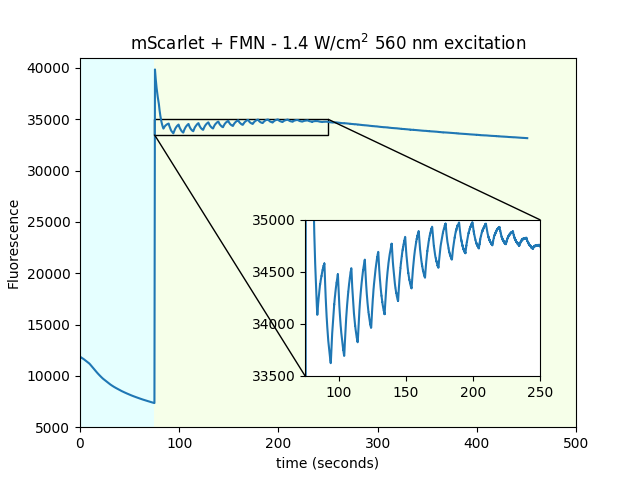
| 550 nm imaging power |
mScarlet magnetofluorescence with non-flavin cofactors
At the suggestion of Adam Cohen, we looked for mScarlet magnetofluorescence with WST-8 as a cofactor instead of FMN. WST-8 is a tetrazolium dye used to assess cellular metabolic activity that forms a 460 nm absorbing formazan dye upon reduction. It works, without needing 470 nm pre-excitation! We also used purified beads coated with mScarlet to screen a library of ~730 metabolites. We screened using 550 nm excitation and a 700 nm emission filter on two 384-well plates. We identified cinnavalininate, an intermediate in tryptophan metabolism, as an additional cofactor. We also ran the screen with 405/470/550/640 nm excitation, and recovered FMN as a positive hit.
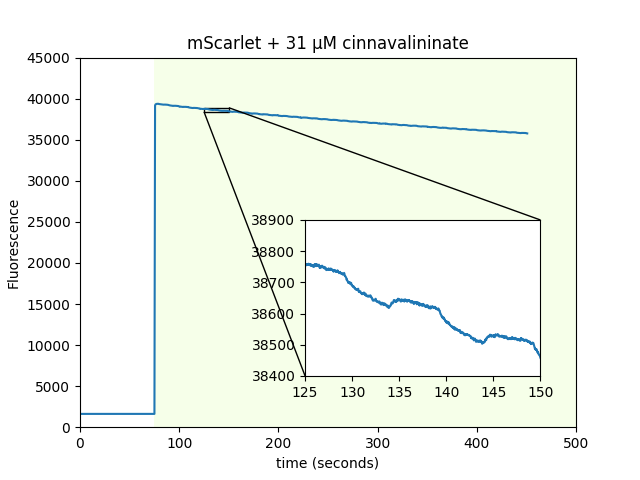
| Sample: |
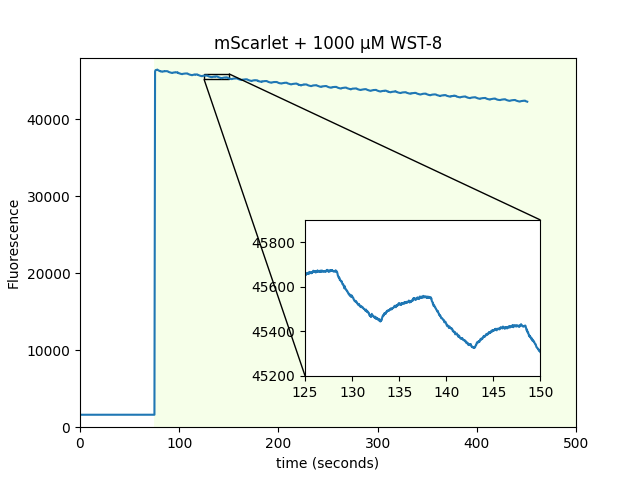
| Sample: |
Additional controls using EGFP immobilized on beads
In order to establish that EGFP is indeed the source of the fluorescent magnetoresponse and it is not merely the flavin accumulating on the bead surface, we deleted tyrosine 66 from EGFP; as shown below, this eliminates magnetoresponse in the green channel. Deletion of the chromophore tyrosine extinguishes the fluorescence while maintaining the overall protein fold, (at least with mVenus, Steven Vogel, personal communication), and we believe it has the same effect on EGFP. We also covalently attached a flavin to the C-terminus of EGFP∆Y66 and observed no modulation by the magnetic field. Unlike EGFP/FAD mixtures, EGFP/FMN mixtures do not exhibit a magnetic field response, possibly because FMN does not bind EGFP.
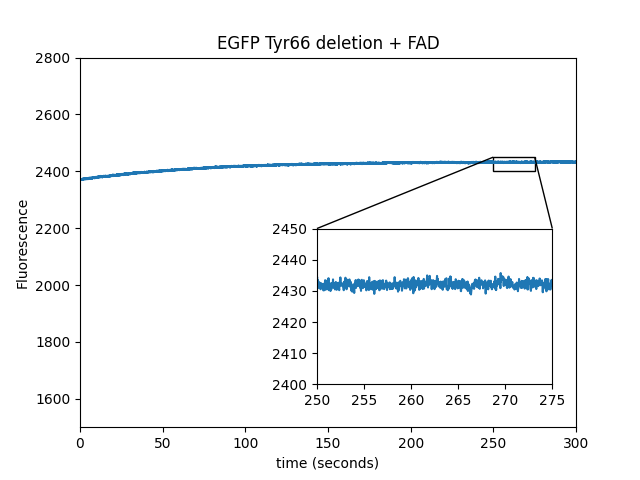
| Sample: |
Additional controls using purified EGFP in solution
We repeated the experiments from Figure 1 (which used immobilized EGFP on beads) using pure protein in solution (PBS), in wells small enough to be completely covered in an FOV. This prevents diffusion artifacts from complicating the interpretation of the traces. We observed similar results, indicating that the beads are irrelevant to the modulation of fluorescence by the magnetic field.
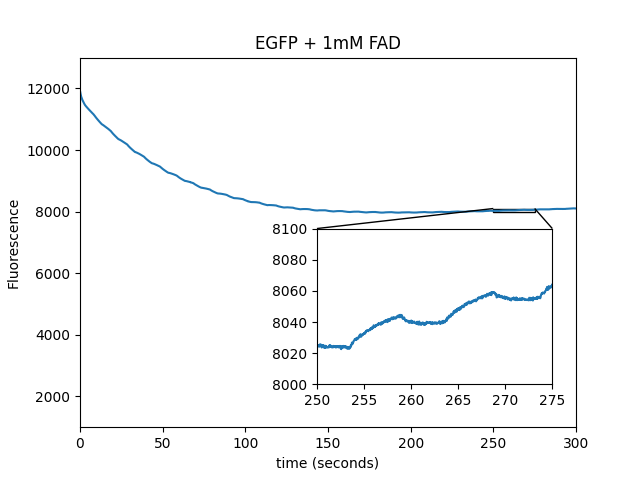
| Sample |
Dependence of EGFP magnetofluorescence on FAD concentration
We used purified EGFP in solution to determine the magnetofluorescent response as a function of FAD concentration.
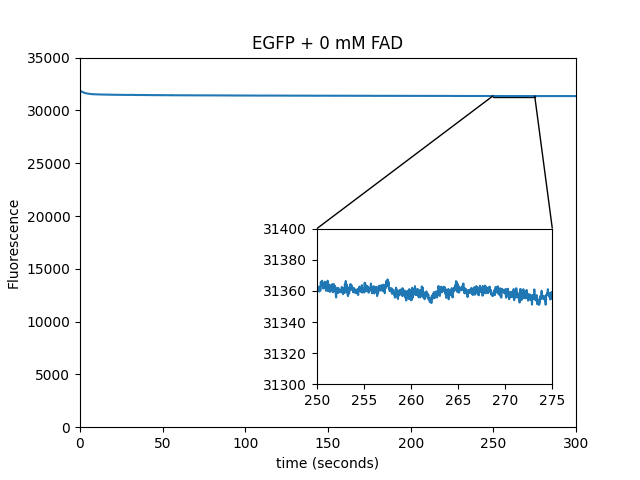
FAD concentration:
Dependence of EGFP magnetofluorescence on 470 nm power
We used 19.1 μM EGFP + 1 mM FAD in solution to determine the dependence of the magnetic field modulation of fluorescence as a function of 470 nm excitation power.
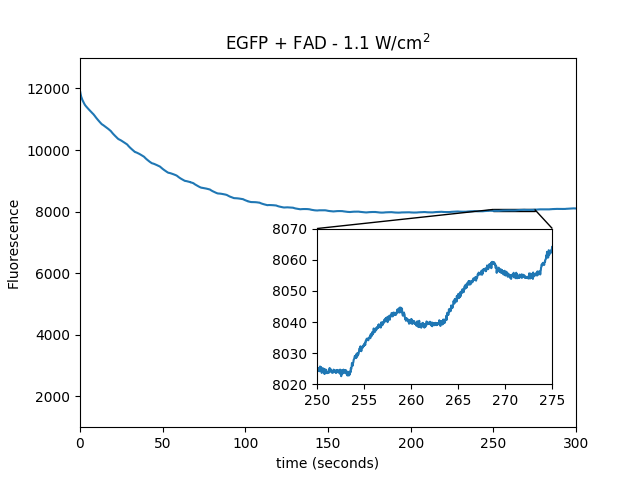
| power: |
EGFP-FlavingTag is magnetoresponsive
We purified beads with a flavin covalently attached to EGFP by a FlavinTag sequence and co-expression of the ApbE flavin transferase. The sequence between the EGFP C-terminus and the FlavinTag also encoded for a TEV protease digestion site. This sample was magnetoresponsive, and TEV protease abolishes the response.
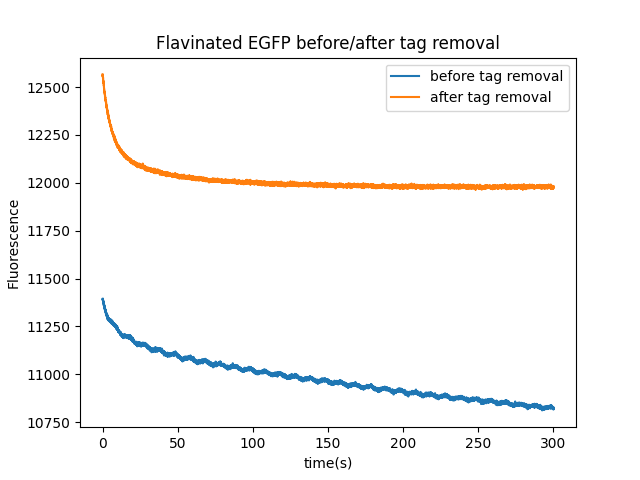
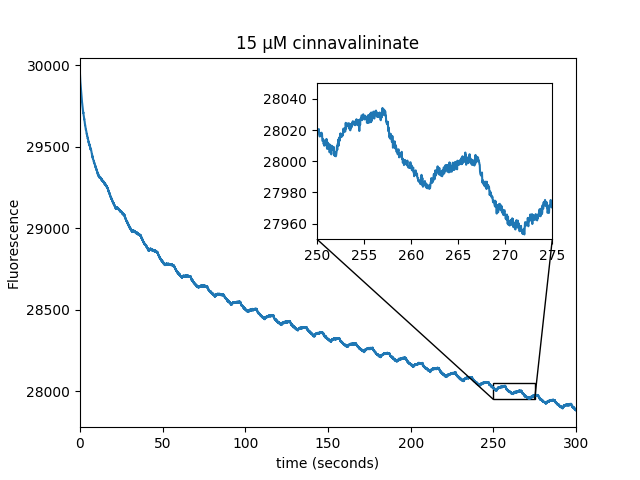
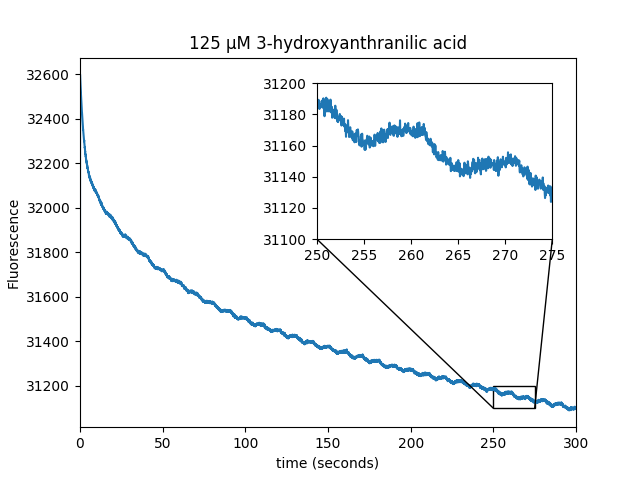
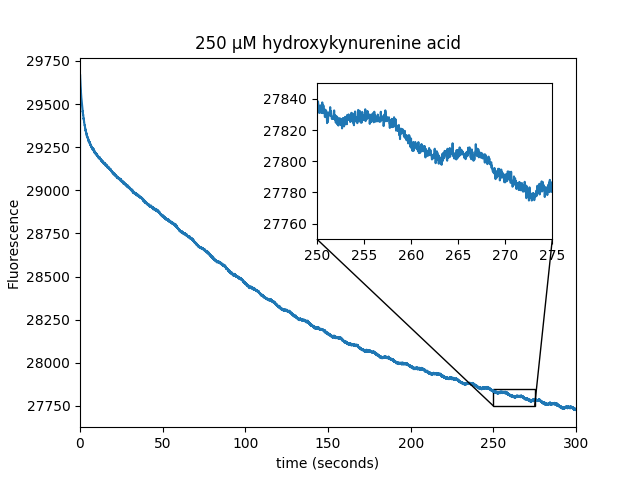
EGFP magnetofluorescence with non-flavin cofactors
We observed that WST-8 dye is able to induce magnetic field modulation of EGFP fluorescence. The metabolite screen also identified several positive hits (cinnavalininate, Hydroxykynurenine, Homogentisic acid, 3-Hydroxyanthranilic acid) as well as recovering FAD as a positive hit.
| Sample: |
| Sample: |
| Sample: |
| Sample: |
List of screened compounds:
Notes on the directed evolution of MagLOV
We engineered MagLOV using the same strategy we used to engineer pH-Countdown [Peksağlam Seidel 2021]. We encourage you to read the linked section of that publication if you're interested in what we tried, and what we learned.
Briefly, semi-random mutagenesis at every residue is our preferred method for protein engineering. We ordered 284 primers (~$1000), and used them to make all single mutants of AsLOV2 C450A at all locations (2840 mutants). We'd then pool the mutants into a single library, spread the library across 5-10 plates and screen for a winner. After selecting a winner, it costs ~$60 to update the primers for the new champion, which becomes the starting point for the next round of mutagenesis.
- $1000 on primers may sound expensive, but this works much better than anything else we tried, and is very cheap compared to the cost of labor and the value of our time.
- The size of this library (104 mutants) matches the rough daily throughput of our plate-based screen. In general, the size of one's libraries should relate to the throughput of one's screen.
- We had great results when we simply reused the same primers for multiple consecutive rounds, rather than updating primers at each round. This probably selected against multiple consecutive nearby mutations, but maybe this is good?
- We typically observed the strongest magnetoresponse two days after transformation, which we suspect is due to the E. coli's internal chemical environment. More generally, the amplitude of magnetoresponse depends on many factors, including chemical environment, but also illumination intensity, magnetic field strength, temperature, etc.
Our screen used the same fluorescence photography system from [Peksağlam Seidel 2021]. We added an electromagnet below the sample (Model: KK-P80/10, Kaka Electric), which we turned on and off via an Arduino Uno. For selection, we put agar plates with E. coli directly on top of this electromagnet. For quantification, (e.g. the data in the main text), we transferred the E. coli to nitrocellulose, which allowed better control of the distance between the magnet and the sample.
Plasmids and sequences
pRSETB 6xHis-mEGFP-FlavinTag plasmid Snapgene file. E. coli plasmid encoding a fusion between 6xHIS-EGFP and the FlavinTag; plain text format
pRham-Streptag-APBE plasmid Snapgene file. E. coli plasmid encoding a flavin transferase; plain text format
pCDH EF1 Mito-EGFP-FLVN-T2A-APBE Snapgene file. Mammalian cell plasmid encoding EGFP-FlavinTag-T2A-ApbE targeted to mitochondria via fusion to the COX8A presequence; plain text format
pRSET-AsLOV2(C450A V416V) Snapgene file. E. coli plasmid encoding a mutant of the LOV domain; plain text format
pRSET B-MagLOV Snapgene file. E. coli plasmid encoding a magnetically sensitive fluorescent protein engineered from AsLOV2; plain text format. Available via Addgene: addgene.org/219957.